This carbocation is also a benzylic carbocation.
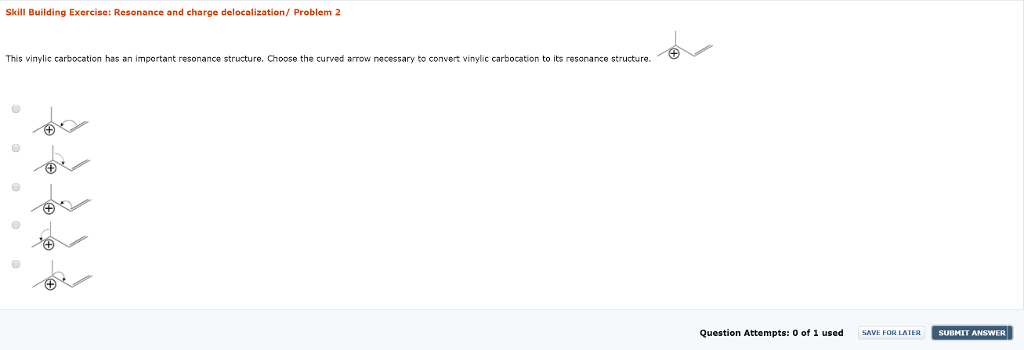
Vinylic carbocation resonance structures.
See also primary allylic carbocation secondary allylic carbocation tertiary allylic carbocation.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
It provides plenty of examples including allylic and vinyli.
In the first mechanism step the alkyne is protonated by hydronium ion a strong acid to produce a resonance stabilized secondary vinylic carbocation shown in red.
The allylic carbon and the nearby double bond.
Formation of the carbocation.
An allylic carbon is one that is directly attached to a pi bond.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
An allylic system has a minimum of 3 carbons.
Carbocation stability resonance rearrangement allylic vinylic examples organic chemistry duration.
This organic chemistry video tutorial explains how to determine which carbocation is most stable.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
When the leaving group leaves the carbon for which it was attached becomes sp 2 hybridized with an empty p.
The organic chemistry tutor 103 594 views 15 37.
The lightest allylic carbocation 1 is called the allyl carbocation.
This delocalization stablizes the allyl carbocation making it more stable than a normal primary carbocation.
Resonance structures allow the charge to be shared among two or more atoms allowing each individual atom to carry a smaller portion of the overall burden.
The true structure of the conjugated allyl carbocation is a hybrid of of the two resonance structure so the positive charge is delocalized over the two terminal carbons.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
Heterolytic bond cleavage results in the ionization of a carbon atom and a leaving group in the starting compound the carbon atom is sp 3 hybridized.
Stability of carbocation intermediates.